Introduction
- Maize is crucial for food security in Sub-Saharan Africa but faces challenges from pests like the fall armyworm (FAW), which causes significant crop damage and yield losses.
- Various strategies to manage FAW have been implemented, but gaps remain in understanding the best application timing and frequency of insecticides, as well as their ecological and economic impacts.
- This study evaluates the effectiveness of synthetic and microbial bioinsecticides against FAW, hypothesizing that early, frequent applications and microbial options may be more sustainable and cost-effective.
- The research aims to provide insights into optimal FAW management practices that balance pest control efficacy with ecological and economic considerations.
Materials and Methods
- Conducted at the University of Lome, Togo, this study explored maize cultivation in a bimodal rainfall region, adapting to year-round farming due to climate variability.
- Utilizing the QPM Obatanpa maize variety, the research employed a 3x4 factorial design in randomized blocks, focusing on optimal planting arrangements and insecticide treatments including EMACOT 019 EC™ and BYPEL 1®.
- Infestation assessments measured FAW larvae numbers and plant damage, while parasitism studies monitored larvae in laboratory to observe parasitoid emergence.
- Economic analysis calculated yield profitability, considering costs and market prices, to evaluate the financial effectiveness of different insecticidal strategies through Benefit-Cost Ratios (BCR) and Return on Investment (ROI).
Results
- Insecticide treatments, particularly emamectin benzoate and a PrGV|Btk blend, did not significantly affect FAW larval populations at early growth stages (V3, V5) but showed a marked reduction in larval numbers and associated plant damage at middle stages (V7, V9).
- Preventive and targeted applications of insecticides were more effective in reducing FAW damage and larval populations compared to a single early application.
- Parasitism rates of FAW larvae varied across growth stages, with early insecticide treatments leading to higher parasitism rates, particularly between V5 and VT.
- Maize grain yield significantly increased in plots receiving preventive and targeted insecticide applications.
- "Preventive" insecticide treatment consistently yields highest gross revenues for Fall Armyworm control.
- "Targeted" and "Preventive" applications show significantly higher benefit-cost ratios compared to other treatments, particularly with PrGV|Btk application.
Discussion
- Both emamectin benzoate and PrGV|Btk effectively reduced FAW larval infestations in maize, with significant larval count reductions observed from the V7 growth stage onwards.
- "Preventive" and "Targeted" treatments demonstrated superior effectiveness in reducing larval damage compared to "Early" treatments, highlighting the importance of not only the timing but also the frequency of insecticide applications in managing FAW damage.
- "Targeted" treatments, which were associated with increased parasitism rates at the V7 stage, suggest an ecological benefit by potentially enhancing biological control of FAW larvae.
- Strategic cessation “Early” application of insecticides can positively influence the biological control of FAW, with bioinsecticides like PrGV|Btk maintaining moderate parasitism rates and suggesting a balance between chemical control and conservation of natural enemies.
- The reduction of FAW infestations, particularly through "Targeted" treatments during critical growth stages (V7 to V9), is essential for maintaining economically viable maize yields, indicating a synergistic potential between effective pest management and crop productivity.
Scope Limitations
- The results of the study are based on experimental setups in specific locations, which may not accurately represent other geographic areas with different climates, soil types, or FAW populations. This limits the ability to generalize findings across different agricultural contexts.
- The study might have focused predominantly on larval stage of the FAW lifecycle, potentially overlooking the effects of insecticide treatments on other stages that could also impact overall pest management effectiveness.
- While the study considers ecological impacts, such as parasitism rates, it may not fully account for broader environmental consequences of the insecticide applications, including effects on other non-target species, and soil health.
- The study was conducted over a specific timeframe that may not capture the variability in FAW infestation patterns or the long-term efficacy and impact of the insecticide treatments across multiple growing seasons.
Abstract
Fall Armyworm (FAW), Spodoptera frugiperda (Lepidoptera: Noctuidae) , is a significant pest causing substantial economic losses in invaded regions, particularly in Sub-Saharan Africa. The prevalent strategy for managing FAW involves insecticide applications, ranging from synthetic to botanical and microbial agents. However, the ecological and economic impacts of these interventions often remain unassessed. This study scrutinizes the ecological and economic viability of two insecticidal treatments: one based on emamectin benzoate and another comprising a combination of Pieris rapae granulovirus (PrGV) and Bacillus thuringiensis subsp. kurstaki (Btk) with varied application timings and frequencies. Both insecticide types were effective in reducing FAW larval populations and the associated crop damage. From an ecological standpoint, the PrGV|Btk treatment enhanced parasitism rates, especially when applied only at the early stage of the crop. However, this approach did not significantly lower crop damage compared to a "targeted" strategy, where insecticide application was contingent upon observed injury levels. Remarkably, the "targeted" strategy led to increased FAW larval parasitism, particularly at the V7 growth stage of the crop. Economically, the "targeted" insecticide application emerged as both effective and efficient, minimizing the need for multiple sprays and thus recommended for managing FAW infestations while considering cost and ecological balance.
Introduction
In the agricultural tapestry of Sub-Saharan Africa (SSA), maize (Zea mays L.) emerges as an important crop, integral to the sustenance, economic vitality, and cultural heritage of countless communities (Alene et al., 2009; Chivenge et al., 2015; Erenstein et al., 2022). Maize cultivation provides food security for millions, supporting livelihoods of smallholder farmers and contributing significantly to the agricultural economy (Alene et al., 2009). Yet, the path to successful maize cultivation in SSA is beset with myriad challenges. From soil-related issues to unpredictable climate patterns and the onslaught of pests (Goergen et al., 2016; Adem et al., 2023; Habte et al., 2023), each factor significantly hampers the productivity of maize and, by extension, the food security and economic resilience of SSA. Among the spectrum of biotic threats, the invasion by the fall armyworm (FAW), Spodoptera frugiperda (Lepidoptera: Noctuidae), emerges as a particularly daunting challenge.
Originating from the tropical and subtropical regions of the Americas (Spark, 1979), FAW made its unwelcome debut in Africa nearly a decade ago (Goergen et al., 2016). Its rapid spread across diverse African landscapes has been relentless, breaching geographical and climatic boundaries to pose a continuous threat to maize production across the continent (Koffi et al., 2020a, 2021, 2023a; Niassy et al., 2021). The larvae of FAW are particularly devastating, and causing significant damage to maize, which can lead to substantial yield losses (Spark, 1979). The voracious larvae have a wide host range but show a strong preference for poaceae plants, especially maize which is more vulnerable (Signoretti et al., 2012; Montezano et al., 2018). Their ability to cause extensive damage in a short period underscores the urgency of finding effective management strategies.
In response to FAW threat, a diverse range of management strategies has been implemented, from traditional cultural methods to advanced biological and chemical controls (Midega et al., 2018; Akutse et al.,2019; Babendreier et al., 2020; Aniwanou et al., 2021; Otim et al., 2021; Nboyine et al.,2022; Agboyi et al., 2023; Chawanda et al., 2023; Fiaboe et al., 2023a, 2024). Among these strategies, biological control, particularly the recruitment of natural enemies such as parasitoids, stands out as a cornerstone of sustainable pest management (Agbodzavu et al., 2018; Koffi et al., 2020b; 2023a; Abang et al., 2021b; Koffi et al., 2023). These approaches are often advocated for incorporation into Integrated Pest Management (IPM) schemes, which seek to harmonize effective pest mitigation with the preservation of environmental health. Yet, finding a fully sustainable combines remains challenging, due to variation in environmental contexts across ecozones.
A fundamental yet often overlooked element of IPM is the strategic timing and frequency of interventions, which are essential for its success (Tang et al., 2010; McClure et al., 2023). However, the effectiveness of IPM can be greatly improved when pest control measures are synchronized with the life cycle of the pest, which is intrinsically linked to crop phenology (Fiaboe et al., 2023b; Idrees et al., 2023). This approach is not only focused on direct pest control; it also considers the broader ecological context, particularly the trophic interactions between pests, their natural enemies, and the host plants. Herbivores, such as pests, have life cycles that are closely tied to the phenological stages of crops, upon which they feed. In turn, the survival and efficacy of natural enemies, mainly parasitoids, depend on the availability and vulnerability of these herbivores (Ratto et al., 2022; Fiaboe et al., 2023b). By carefully planning pest management activities to coincide with these interconnected life cycles, IPM strategies not only target pests more effectively but also help preserve and support the beneficial organisms that play a vital role in the natural regulation of pest populations.
On the other hand, the economic ramifications of different control strategies against FAW in SSA remain underexplored. Meanwhile, by targeting pest control measures to coincide with key points in the pest and crop life cycles, farmers can optimize the use of resources, reducing the need for frequent or excessive chemical applications (van den Berg et al., 2021; Nboyine et al., 2022). This targeted approach may minimize input costs and maximizes yield potential by ensuring crops are protected at their most vulnerable stages.
This study sought to bridge these gaps by examining the efficacy of synthetic emamectin benzoate and microbial bioinsecticides (Pieris rapae Granulosis Virus [1] and Bacillus thuringiensis subsp. kurstaki), under on-station experiment conditions. The study hypothesized that the timing and frequency of insecticide applications play a critical role in managing FAW larval outbreaks. To test this, the study compared the ecological and economic impacts of various application strategies: early treatment (applying insecticides at the initial growth phase of maize), late treatment (applying at the advanced vegetative stage of maize), preventive treatment (regular applications based on a predetermined schedule), and targeted treatment (applying based on observed damage levels). Additionally, an economic evaluation was carried out to ascertain the cost-benefit ratio of each method, with the hypothesis that an infestation-level-driven approach to insecticide application would strike a balance between effectiveness and economic feasibility. This approach was proposed as a sustainable method for controlling FAW.
[1] PrGV is a baculovirus targeting cabbage white butterfly larvae, used in biocontrol of agricultural pests (Zhang et al., 2012).Materials and Methods
Study site
The study was conducted at the agricultural research facility of the University of Lome, Togo, positioned at 6°22'N latitude and 1°13'E longitude, with an elevation of 50 meters. The research site falls within the Coastal Savannah Agro-ecological Zone, which is known for its bimodal distribution of rainfall. The initial rainy phase extends from the middle of March to the end of July, succeeded by a second phase of rainfall that spans from the start of September to the middle of November (Fiaboe et al., 2024). Traditionally, maize cultivation aligns with two distinct seasons, from April to July and from September to November, benefiting from an average yearly rainfall ranging between 800 mm and 1100 mm, alongside a mean temperature of 27°C annually. The observed shifts in climate patterns and a continuous need for maize have led to year-round cultivation practices by local farmers, moving away from conventional seasonal cycles (Fiaboe et al., 2024). Prior to this study, the experimental field was left fallow for a year and then used for maize cultivation. The predominant soil type at the site is classified as ferralsol.
Experimental design and treatments
Two on-station experiments were conducted from April 10 to July 29, 2021, and September 26, 2021, to January 14, 2022, respectively. Certified maize seed variety QPM Obatanpa, supplied by the Togolese Institute of Agronomic Research (ITRA) was used. This particular variety of maize typically reaches maturity approximately 105 days following planting (Fiaboe et al., 2024). The experimental framework was structured as a 3x4 factorial design within a randomized block setup, including four replicates.
Planting was organized in a pattern of 0.80 m between rows and 0.20 m between plants, ensuring one plant per hill. Each experimental plot measured 5.6x6 m, with a 3-m buffer alley between both blocks and individual units to prevent treatment interference. Nutrient management included the application of a complex N15P15K15 fertilizer, administered at a rate of 250 kg/ha fifteen days following seedling emergence, supplemented by an additional 100 kg of nitrogen per hectare in the form of ammonium nitrate after 30 days. The experimental plots were maintained following standard agricultural practices, which encompassed manual, human-powered weed control.
The insecticidal treatments involved EMACOT 019ECTM, containing 19.2 g/L of emamectin benzoate, and BYPEL 1®, a blend of Pieris rapae granulovirus (PrGV) at 10,000 PIB/mg and Bacillus thuringiensis subsp. kurstaki (Btk) at 16,000 IU/mg. Insecticide dosages adhered to recommended guidelines, employing a 1 mL/L water solution for EMACOT 019 ECTM and a 1.33 g/L water solution for BYPEL 1®, ensuring application volumes of 350 L/ha and 200 L/ha, respectively. To ensure optimal efficacy, all insecticide applications were carried out in the late afternoon, specifically between 17:00 and 18:00 hrs, using 16-L Ingco backpack sprayers dedicated separately for the synthetic and microbial insecticide treatments to prevent cross contamination.
We assessed four distinct application frequencies: an "early" treatment involving a single application at 10 days post-emergence (corresponding to the V3 growth stage); a "preventive" strategy with applications at 10 days post-emergence followed by additional treatments at the V5, V7, and V9 stages; a "targeted" approach applying insecticide based on visual damage assessments exceeding predefined thresholds (Prasanna et al., 2018); and a "late" intervention at the V9 growth stage. Growth stages were determined using the Iowa State University scaling system (Infante et al., 2018), which identifies stages from the third collar leaf (V3) to the nth collar leaf (Vn), along with tasseling (VT), milk (R3) stage, and the dry cobs stage.
Infestation and parasitism
Data on infestation were collected, focusing on two primary metrics: the extent of damage to each maize plant and the count of FAW larvae present. These observations were recorded at four growth stages of the maize plants: V3, V5, V7, and V9. A destructive sampling method was employed, where five plants from each treatment plot were carefully selected and severed at the base. These plants were then transported to the Entomology and Nematology Laboratory at the School of Agronomy (ESA), University of Lome, for further analysis. The assessment of damage to the plants was conducted using the scoring system developed by Davis et al. (1992), which ranges from 0 (indicating no damage) to 9 (indicating the highest level of damage), as detailed in Table S1. Simultaneously, larvae were carefully counted from each plant to gauge the severity of infestation.
For parasitism analysis, observations were extended to include the VT, R3, and dry cob stages of maize growth. The larvae collected were individually placed in 100 mL clear plastic cups (Everpack Ghana Ltd, Accra, Ghana) with aeration to monitor parasitoid emergence. The cups were maintained at 26±1°C, 75-80% relative humidity, and a 12:12-hour light-dark photoperiod. To track parasitoid emergence, larvae were fed insecticide-free fresh maize leaves daily. The identification of parasitoids emerging from the larvae was conducted by the last two authors of this paper, drawing upon their taxonomical expertise in the species (Koffi et al., 2020b; 2023a).
Yield and economic analysis
Maize cobs were harvested manually from each plant when they reached maturity, 110 days after sowing. After harvesting, the cobs were laid out to dry in the sun for 12 days. After proper drying, the cobs were shelled to separate the grains, which were then weighed. Weight measurements were collected from groups of 10 plants per experimental plot to ensure a representative sample. The weights were scaled up to hectare-level yields, accounting for the plant density used in the experimental design.
The yield values were used in the economic analysis using the following formulas (Mauki et al., 2023) to determine:
Gross revenue per hectare (GR), calculated using:GR = Yield(kg/ha) x Market price(USD/kg)
(1)The market favored control and PrGV|Btk-treated maize with higher prices compared to those treated with emamectin benzoate, reflecting the premium on ecologically-produced maize over synthetic alternatives (Table S2).
Net profit (NP) per hectare was then calculated as:
NP = GR - TC
(2)where TC encompasses the sum of:
- Seed price per hectare
- Manure cost per hectare
- Sowing labor cost per hectare
- Insecticide costs, calculated as the product of the number of applications and the sum of insecticide price, water cost, and labor cost per event.
The economic viability was assessed via Benefit-Cost Ratio (BCR):
BCR = GR / (TC)
(3)A BCR above 1 implies profitability, while below 1 suggests a loss.
Lastly, Return on Investment (ROI) was quantified to determine the investment's profitability, calculated as:
ROI = (NP x 100) / TC
(4)Data analysis
Before performing statistical analyses, data were assessed for normality using the Shapiro-Wilk test, and for homogeneity of variances with the Levene's test. Upon satisfying these preliminary conditions, larvae count data were analyzed using a Likelihood Ratio Test (LR test) within a Generalized Linear Model (GLM) framework, incorporating likelihood radio test (LR test) in the log link function. Damage scores, maize grain yield, and economic data were evaluated using a One-Way Analysis of Variance (ANOVA), with "Best Model" serving as the model selection criterion and Mean Squared Error (MSE) as the performance metric. Partial Least Squares Discriminant Analysis (PLS-DA) was employed to examine correlations between insecticide application strategies and the collected biological and ecological data.
For all statistical tests, a significance level of P<.05 was established. Tukey's Honestly Significant Difference (HSD) test facilitated post-hoc mean comparisons, adhering to a significance threshold of P≤.05. Two-sample t-test was used to compare two independent samples, with alternative hypothesis set at Mean 1 − Mean 2 ≠ 0. All analyses were performed using RStudio version 4.1.2 (R Core Team, 2022).
Results
Influence of insecticide treatments on FAW larvae
In the first experiment, FAW larvae counts at the maize V3 growth stage did not significantly differ among treatment groups (LR-GLM, χ2=0.81, df=4, P>.05; Table 1), a trend that was consistent in the subsequent trial (LR-GLM, χ2=1.20, df=4, P>.05; Table 1). Neither emamectin benzoate nor PrGV|Btk treatments significantly impacted FAW populations at the V5 stage in both the first (LR-GLM, χ2=9.00, df=4, P>.05; Table 1), and second (LR-GLM, χ2=4.88, df=4, P>.05;Table 1) experiments. However, in the second experiment, a marked decrease in FAW larvae per plant was noted at the V7 stage, especially in plots receiving preventive or targeted applications of emamectin benzoate (LR-GLM, χ2=14.08, df=4, P<.01; Table 1), and PrGV|Btk (LR-GLM, χ2=25.00, df=4, P<.0001; Table 1) treatments. Similarly, in the second experiments, at the V9 stage, a significant reduction in FAW larvae was observed in treated plots, particularly those under "preventive" and "targeted" applications of emamectin benzoate (LR-GLM, χ2=22.15, df=4, P<.001; Table 1) and PrGV|Btk (LR-GLM, χ2=11.69, df=4, P<.05; Table 1).
At the VT stage of maize development, the "Preventive" treatment plots exhibited the lowest counts of FAW larvae, with significant reductions observed in both the initial (LR-GLM, χ2=16.62, df=4, P<.05; Table 1), and subsequent (LR-GLM, χ2=12.80, df=4, P<.05; Table 1) experiments. However, by the R3 stage, no significasnt differences in larval counts were detected among the various treatment groups in the second trial, regardless of the type of insecticide applied (LR-GLM, χ2=6.82, df=4, P>.05; Table 1). This lack of significant difference persisted in treatments using emamectin benzoate and PrGV|Btk, as evidenced by the FAW larvae counts per maize cob (LR-GLM, χ2=1.93 and χ2=0.85, respectively, df=4, P>.05; Table 1).
| Crop Stage | Insecticide | Insecticide application regimes | P-value | ||||
|---|---|---|---|---|---|---|---|
| Control | Early | Preventive | Targeted | Late | |||
| First Experiment | |||||||
| V3 | Em. benz. | 2.5±0.26a | 2.9±0.34a | 3.1±0.34a | 3.1±0.36a | 2.7±0.32a | .851ns |
| PrGV|Btk | 2.5±0.26a | 2.8±0.36a | 2.7±0.36a | 2.9±0.31a | 3.0±0.28a | .948ns | |
|
|
|
|
|||||
| V5 | Em. benz. | 1.9±0.23a | 1.4±0.21a | 1.4±0.22a | 1.3±0.16a | 1.9±0.25a | .285ns |
| PrGV|Btk | 1.9±0.23a | 1.7±0.21a | 1.2±0.19a | 1.6±0.21a | 2.1±0.22a | .174ns | |
|
|
|||||||
| V7 | Em. benz. | 1.2±0.22a | 1.3±0.22a | 0.85±0.19a | 1.0±0.16a | 1.2±0.21a | .619ns |
| PrGV|Btk | 1.2±0.22a | 0.5±0.15a | 0.80±0.18a | 0.9±0.14a | 1.3±0.23a | .072ns | |
|
|
|
|
|||||
| V9 | Em. benz. | 1.2±0.18a | 0.9±0.16a | 0.5±0.13a | 0.3±0.12a | 0.8±0.16a | .089ns |
| PrGV|Btk | 1.2±0.18a | 0.6±0.15a | 0.4±0.13a | 0.1±0.06a | 0.6±0.13a | .069ns | |
|
|
|||||||
| VT | Em. benz. | 0.8±0.16a | 0.6±0.15a | 0.3±0.12a | 0.4±0.15a | 0.2±0.11a | .053ns |
| PrGV|Btk | 0.8±0.16c | 0.6±0.19bc | 0.1±0.06a | 0.2±0.11a | 0.4±0.10ab | .003** | |
|
|
|||||||
| R3 | Em. benz. | 0.6±0.11a | 0.6±0.15a | 0.4±0.13a | 0.4±0.13a | 0.3±0.12a | .308ns |
| PrGV|Btk | 0.6±0.11a | 0.5±0.18a | 0.1±0.08a | 0.2±0.12a | 0.3±0.10a | .056ns | |
|
|
|||||||
| Cobs | Em. benz. | 0.6±0.23a | 0.7±0.22a | 0.3±0.10a | 0.3±0.12a | 0.4±0.15a | .505ns |
| PrGV|Btk | 0.6±0.23a | 0.7±0.17a | 0.3±0.14a | 0.2±0.12a | 0.5±0.13a | .314ns | |
|
|
|||||||
| Second Experiment | |||||||
| V3 | Em. benz. | 2.4±0.31a | 2.5±0.31a | 2.8±0.31a | 2.6±0.41a | 2.6±0.43a | .933ns |
| PrGV|Btk | 2.4±0.31a | 2.3±0.36a | 2.4±0.33a | 2.9±0.34a | 2.6±0.43a | .933ns | |
|
|
|
|
|||||
| V5 | Em. benz. | 2.2±0.27a | 1.8±0.20a | 1.8±0.26a | 1.6±0.27a | 2.2±0.21a | .616ns |
| PrGV|Btk | 2.2±0.27a | 2.1±0.22a | 1.6±0.24a | 1.5±0.21a | 2.2±0.21a | .616ns | |
|
|
|
||||||
| V7 | Em. benz. | 1.3±0.19b | 1.1±0.23ab | 0.5±0.11a | 0.5±0.16ab | 1.2±0.18ab | .007** |
| PrGV|Btk | 1.3±0.19b | 0.6±0.16b | 0.4±0.13a | 0.4±0.15a | 1.4±0.19b | <.001*** | |
|
|
|
||||||
| V9 | Em. benz. | 1.0±0.17bc | 1.1±0.21c | 0.1±0.07a | 0.9±0.22bc | 0.6±0.14b | <.001*** |
| PrGV|Btk | 1.0±0.17c | 0.5±0.11ab | 0.2±0.12a | 0.4±0.15ab | 0.6±0.15b | .001*** | |
|
|
|
||||||
| VT | Em. benz. | 0.6±0.27a | 0.6±0.26a | 0.4±0.19a | 0.5±0.19a | 0.5±0.18a | .867ns |
| PrGV|Btk | 0.6±0.27bc | 0.9±0.27c | 0.1±0.07a | 0.2±0.11ab | 0.6±0.21bc | <.001*** | |
|
|
|||||||
| R3 | Em. benz. | 0.4±0.19a | 0.5±0.18a | 0.2±0.11a | 0.3±0.12a | 0.2±0.12a | .449ns |
| PrGV|Btk | 0.4±0.19a | 0.3±0.15a | 0.3±0.12a | 0.1±0.07a | 0.2±0.08a | .544ns | |
|
|
|||||||
| Cobs | Em. benz. | 0.4±0.19a | 0.5±0.18a | 0.2±0.11a | 0.3±0.12a | 0.2±0.12a | .748ns |
| PrGV|Btk | 0.4±0.19a | 0.3±0.15a | 0.3±0.12a | 0.1±0.07a | 0.2±0.08a | .932ns | |
|
|
|||||||
This table details the mean counts of Spodoptera frugiperda larvae per maize plant recorded at key growth stages during two on-station experiments. The first experiment was conducted from April 10 to July 29, 2021. Growth stages V3, V5, V7, and V9 correspond to vegetative phases inspected on April 20, April 30, May 10, and May 20, respectively. The VT stage (tasseling) was assessed on June 19, while the R3 stage (milk) was evaluated on July 9. The second experiment spanned from September 26, 2021, to January 14, 2022. Vegetative stages V3, V5, V7, and V9 were examined on October 6, October 16, October 26, and November 5, 2021, respectively. The tasseling phase (VT) was observed on November 25, and the milk stage (R3) was inspected on December 15. The "Cobs" category represents the assessment of larval counts on dry maize cobs conducted on January 14, 2022. PrGV denotes Pieris rapae Granulovirus, and Btk represents Bacillus thuringiensis subsp. kurstaki. Displayed values indicate average larval counts alongside the standard error (SE). Data analysis was performed using ANOVA (n=5, selecting the best model based on mean squared error (MSE), with Tukey's HSD test for the differentiation of means. Insecticide application events are marked with brown icons. The statistical significance of the results is denoted by asterisks, with * indicating P < .05, ** indicating P < .01, and *** indicating P < .001; ns indicates no significant difference.
Effect of insecticide treatments on maize plant damage
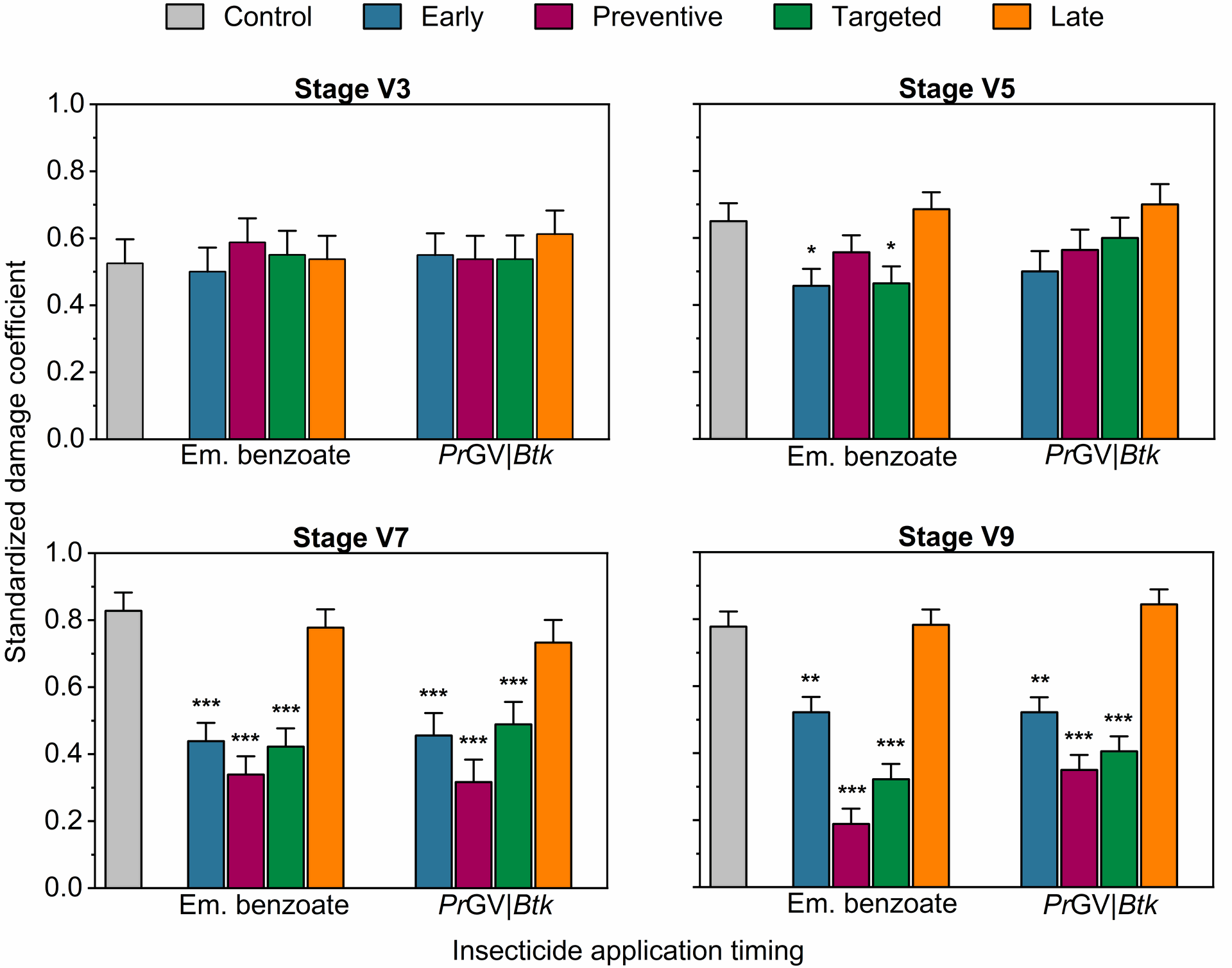
Effect of insecticide treatments on Spodoptera frugiperda-related damage in maize at various growth stages during the first on-station experiment. The bars display the mean±SE damage coefficients, which quantify the extent of damage inflicted by FAW larvae on maize plants at different developmental stages. The damage coefficients are normalized by dividing each plant's damage score (Davis et al., 1992) by the highest score recorded at a particular growth stage. The abbreviations Em., PrGV, and Btk denote emamectin benzoate, Pieris rapae Granulovirus, and Bacillus thuringiensis subsp. kurstaki, respectively. The analysis was conducted using One-Way Analysis of Variance (ANOVA) with a significance level set at α = 5%. The statistical significance of the results is denoted by asterisks, with * indicating P < .05, ** indicating P .01, and *** indicating P < .001.
The observations indicate that the extent of damage inflicted by FAW on maize plants at the V3 growth stage was uniformly distributed across all treatment plots in both on-station experiments (One-Way ANOVA, F4,175=0.44 and F4,175=0.41, respectively, P>.05; Figure 1 & 2). However, the use of emamectin benzoate in the first experiment resulted in significantly reduced damage at the V5 stage (One-Way ANOVA, F4,95=4.01, P<.01; Figure 1), an effect not observed with PrGV|Btk treatments (One-Way ANOVA, F4,175=0.43, P>.05; Figure 1).
Conversely, at the V7 stage, in both experiments, insecticide applications led to significant differences in damage levels (One-Way ANOVA, F4,175=33.41 and F4,175 = 16.84, respectively, P<.0001; Figure 1 & 2), with the lowest damage noted in early, preventive, and targeted treatment plots. This pattern continued at the V9 stage, where the lowest damage was recorded in preventive treatment plots that underwent four insecticide applications, in both experiments (One-Way ANOVA, F4,175=37.51 and F4,175=31.82, respectively, P<.0001; Figure 1 & 2).
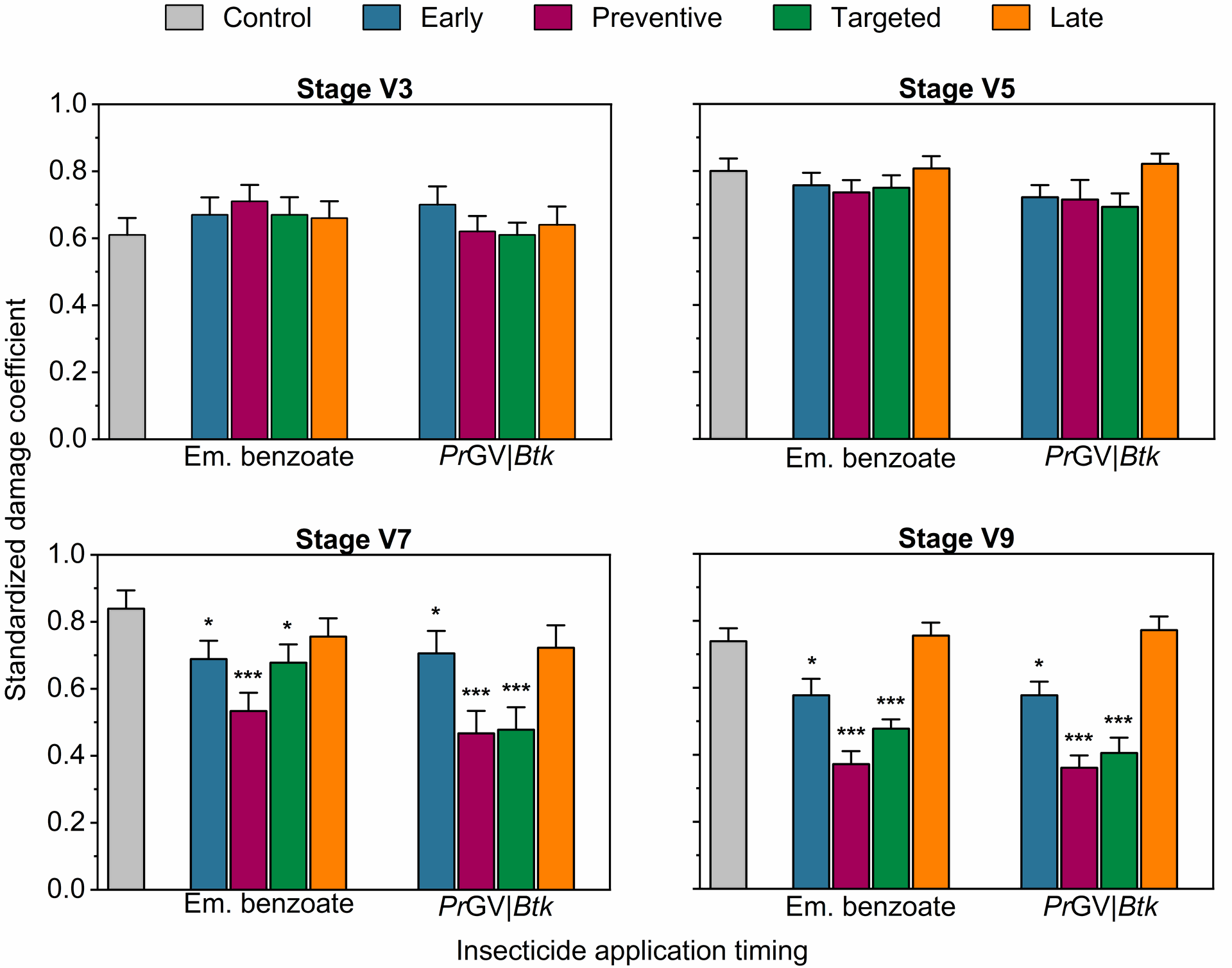
Effect of Insecticide treatments on Spodoptera frugiperda-related damage in maize at various growth stages during the second on-station experiment. The bars display the mean±SE damage coefficients, which quantify the extent of damage inflicted by FAW larvae on maize plants at different developmental stages. The damage coefficients are normalized by dividing each plant's damage score (Davis et al., 1992) by the highest score recorded at a particular growth stage. The abbreviations Em., PrGV, and Btk denote emamectin benzoate, Pieris rapae Granulovirus, and Bacillus thuringiensis subsp. kurstaki, respectively. The analysis was conducted using One-Way Analysis of Variance (ANOVA) with a significance level set at α = 5%. The significance of the results is denoted by asterisks, with * indicating P < .05, ** indicating P < .01, and *** indicating P < .001, highlighting the statistical relevance of the observed differences.
Impact of insecticides on FAW parasitoids
The study reported the occurrence of parasitoids such as Chelonus bifoveolatus Szpligeti (Hymenoptera: Braconidae), Chelonus insularis Cresson (Hymenoptera: Braconidae), Coccygidium luteum (Brullé) (Hymenoptera: Braconidae), and Cotesia sp. (Hymenoptera: Braconidae) in FAW larvae that were incubated for observation (Table S2). The rates of parasitism unveiled significant variation at different growth stages of maize plants (Figure 3). showed the highest parasitism rates during the initial stages of the crop (V3 and V5; Figure 3). Remarkably, in the first experiment, plots that received early insecticide treatments demonstrated the highest rates of parasitism, particularly peaking at the V9 and VT stages, irrespective of the type of insecticide used (Figure 3). Similarly, in both experiments, plots with targeted insecticide treatments, especially when PrGV|Btk was applied, showed a parasitism rate peak between the V7 and V9 stages. For plots where emamectin benzoate was applied preventively, a notable peak in parasitism rates was observed at the VT stage of maize growth.
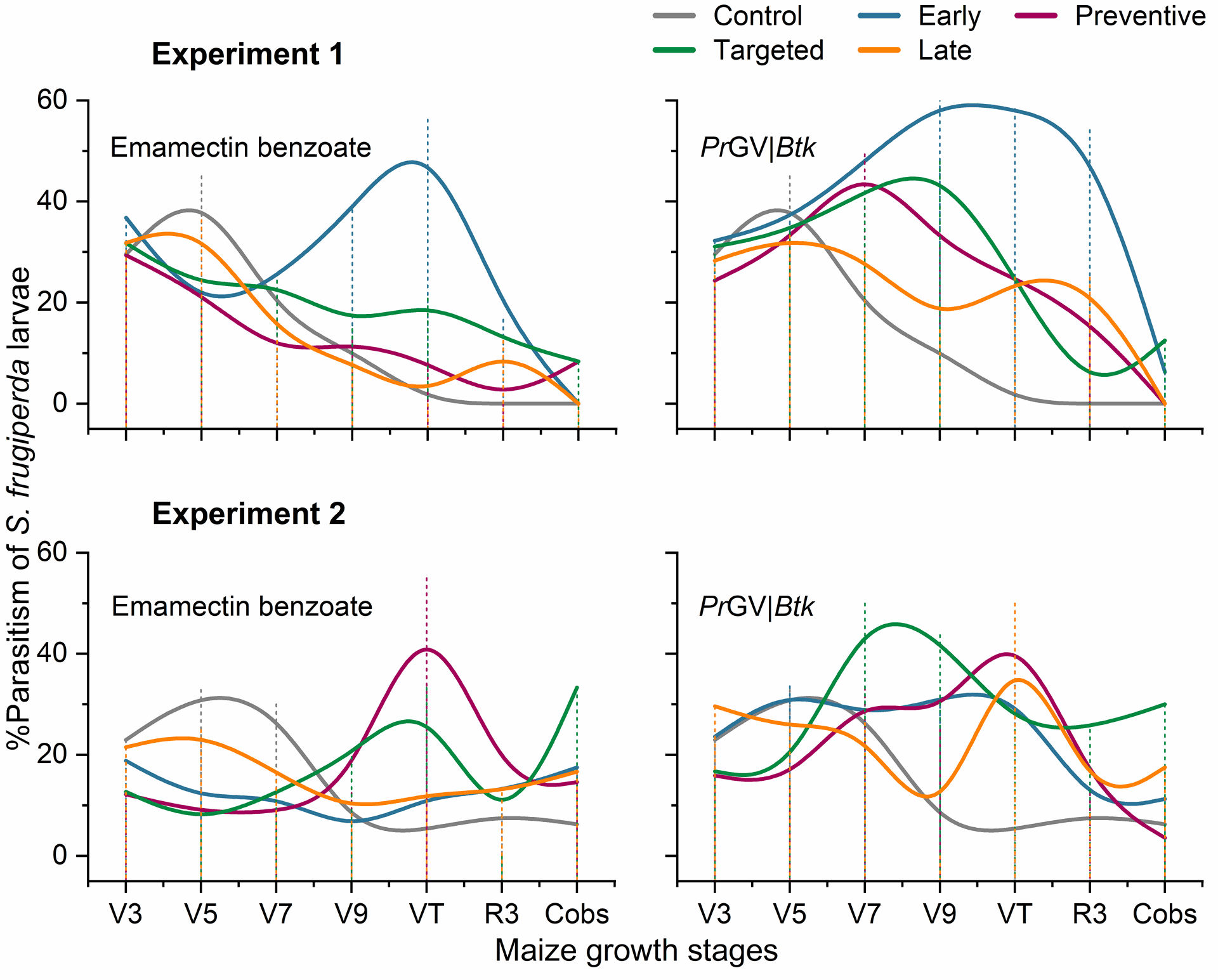
Effect of insecticide treatments on parasitism rates of FAW larvae. This figure illustrates the changes in parasitism rates of FAW larvae among various treatment plots throughout the maize plant growth stages. The lines trace the parasitism dynamics, reflecting how different insecticide applications influence the interaction between FAW larvae and their parasitoids over time. For specifics on when insecticides were applied for each treatment group, see Figure S1. The abbreviations PrGV and Btk stand for Pieris rapae Granulovirus and Bacillus thuringiensis subsp. kurstaki respectively, indicating the types of bioinsecticides evaluated in this study.
PLS-DA on insecticide strategy variables for FAW management
Partial Least Squares Discriminant Analysis (PLS-DA) is used here to illustrate the biological and ecological variables associated with different insecticide application timings in the context of managing FAW infestation. The plot combines the first (wc1) and second (wc2) latent variables, which together account for 34% of the variance explained by the PLS-DA model (Figure 4).
The results suggest that "Late" and "Control" insecticide application treatments have similar profiles (Figure 4). Conversely, "Preventive" and "Targeted" treatments are associated with another set of variables, with both treatment strategies appearing to be distinct from the "Early" treatment. Remarkably, the biplot reveals that variable such as parasitism of FAW larvae at V7 and “cobs" stages are closely aligned with the "Targeted" treatment strategy, while maize leaf damage at V5, V7, and V9, and larval incidence at V5 and V7 maize stage was associated with the control treatment (Figure 4).
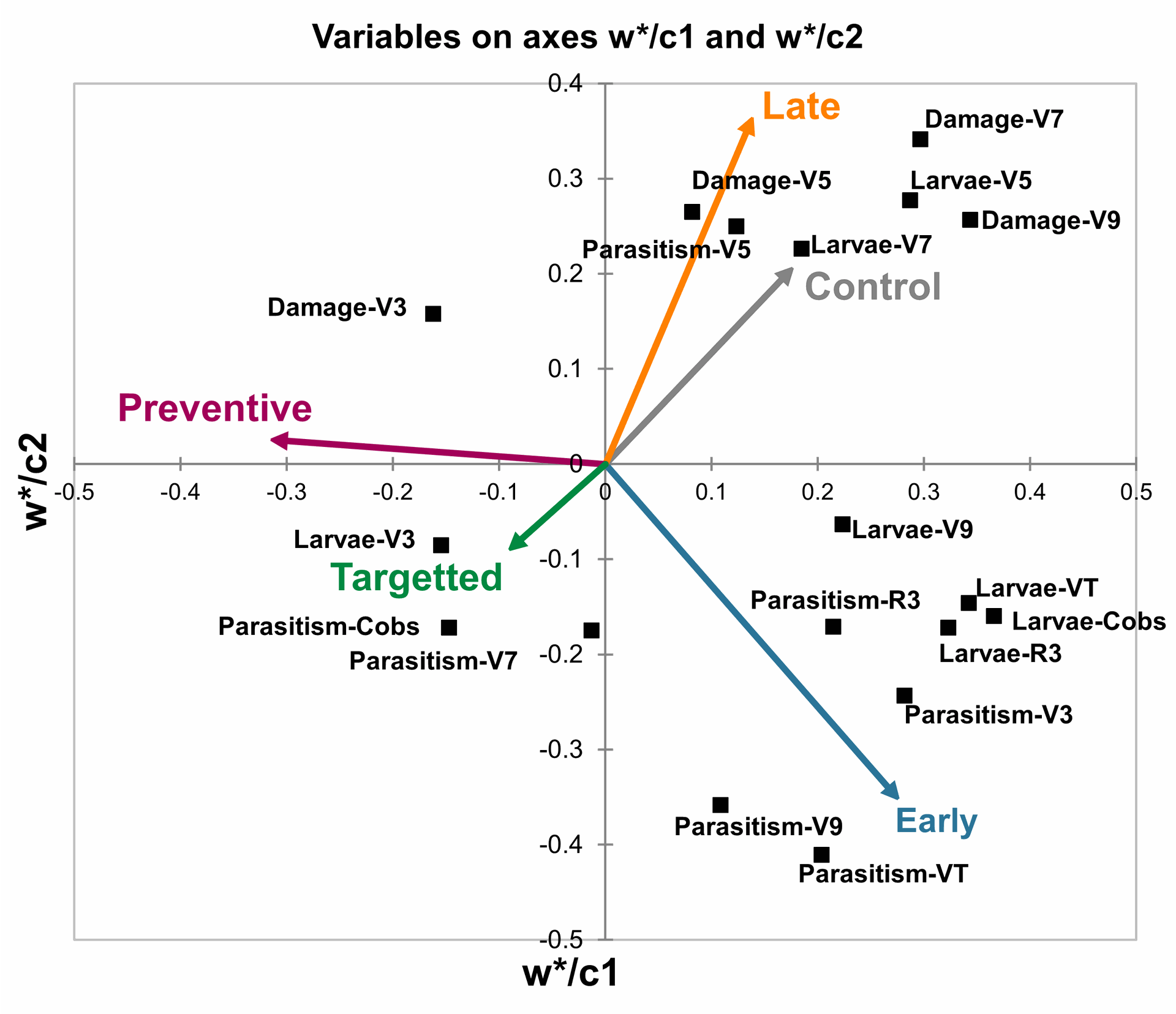
PLS-DA Biplot of Insecticide Application Timings on FAW Management. This biplot illustrates the associations between different insecticide application strategies ('Late', 'Control', 'Preventive', 'Targeted', and 'Early') and key biological and ecological variables in the context of FAW control. The axes represent the first (wc1) and second (wc2) latent variables of the PLS-DA model, explaining 34% of the total variance
Economic efficiency of insecticide control of FAW
According to the economic analyses, the highest gross revenues were consistently observed in the "Preventive" and "Targeted," followed by "Early," and "Late" treatments, both initially and in subsequent experiments (One-Way ANOVA, F4,35=38.95 and F4,35=242.08, respectively, P<.0001; Figure 5A& B; Table S3). However, no statistical difference was found between the "targeted" and "preventive" applications concerning gross revenues in either experiment (t-Test, t=0.613 and t=-0.169, df=6, P>.05; Figure 5A & B).
Interestingly, during the initial experiment, both "targeted" and "Preventive" insecticide application models demonstrated significantly higher revenues for PrGV|Btk treatment over emamectin benzoate (t -Test, t=-5.33 and t=-7.57, respectively, df=6, P<.001; Figure 5A). Similarly, in the second experiment, higher revenues were obtained from PrGV|Btk treatment plots when applied preventively (t-Test, t=-8.62, df=6, P<.001; Figure 5B), or targeted (t-Test, t=-69.53, df=6, P<.0001; Figure 5B).
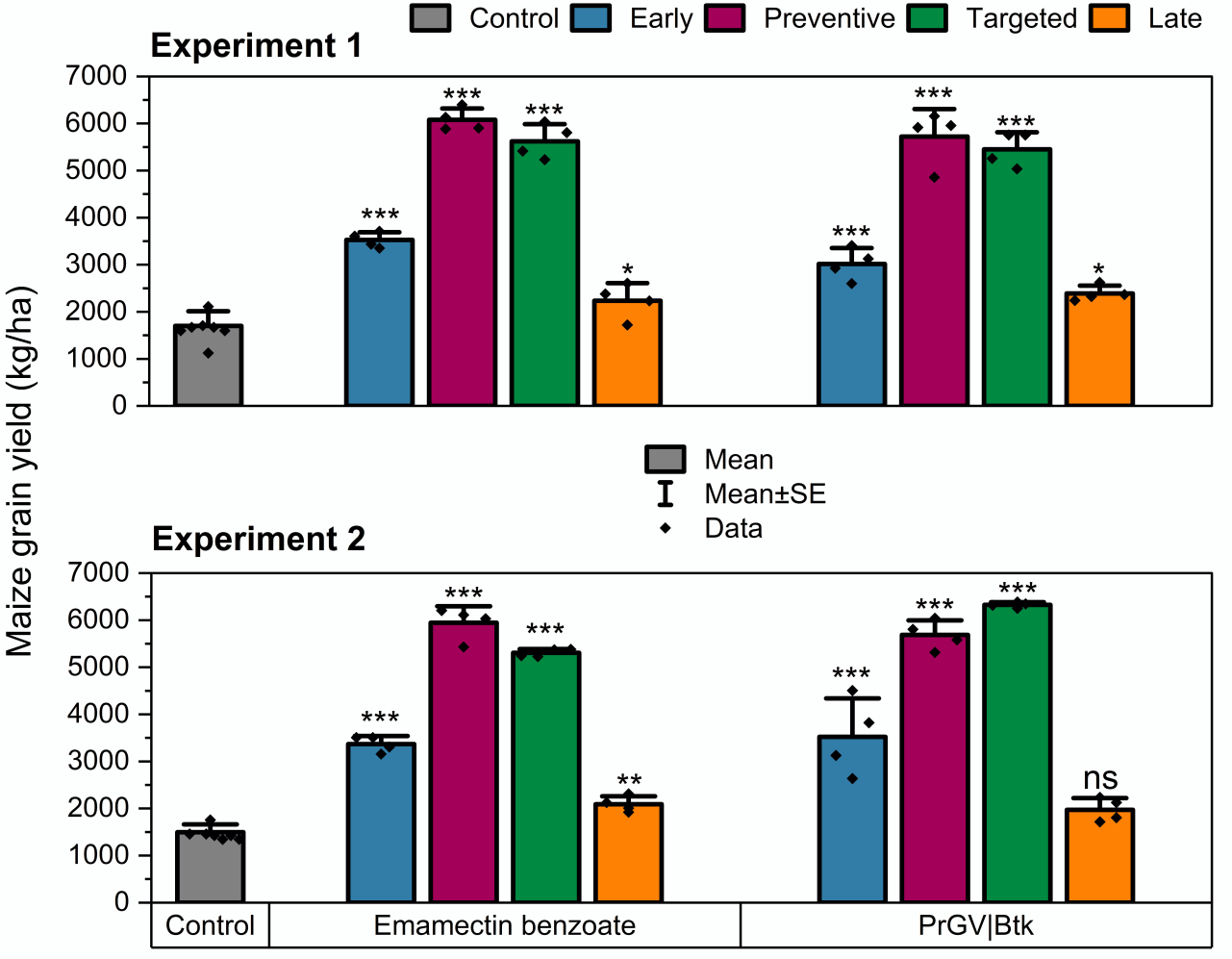
Effects of insecticide on the maize grain yield. Bars depict the mean±SE maize grain yield. The bars represent the average maize grain yield (±SE) across different treatment plots, with yields extrapolated from measurements of 10 plants per plot to tons per hectare (t/ha). The abbreviations PrGV and Btk represent Pieris rapae Granulovirus and Baccilus thuringiensis subsp. kurstaki respectively, indicating the specific bioinsecticides evaluated. Yield data were analyzed using One-Way Analysis of Variance (ANOVA), with a 5% significance threshold (α = .05). The effect of each insecticide treatment on yield, relative to the untreated control, is highlighted by asterisks: * for P < .05, ** for P .01, and *** for P < .001, signifying varying levels of statistical significance. The notation 'ns' indicates no significant difference from the control.
Comparable trends were noted in the BCR values, with all ratios surpassing 1 (Figure 6C&D). Specifically, both "Targeted" and "Preventive" applications consistently demonstrated significantly higher BCRs compared to other treatments, including "Early," "Late," and the control, across both experiments (One-Way ANOVA, F4,35=24.96 and F4,35=22.46, respectively, P<.0001; Figure 6C&D; Table S3). Moreover, consistently higher values were associated with PrGV|Btk application (Figure 6C&D, P<.0001). Notably, the BCR of the "Late" application was either similar to or significantly lower than the control treatment, irrespective of the insecticides used (One-Way ANOVA, F4,15=92.68 and F4,15=92.68, respectively, P<.0001; Figure 6C; Table S3).
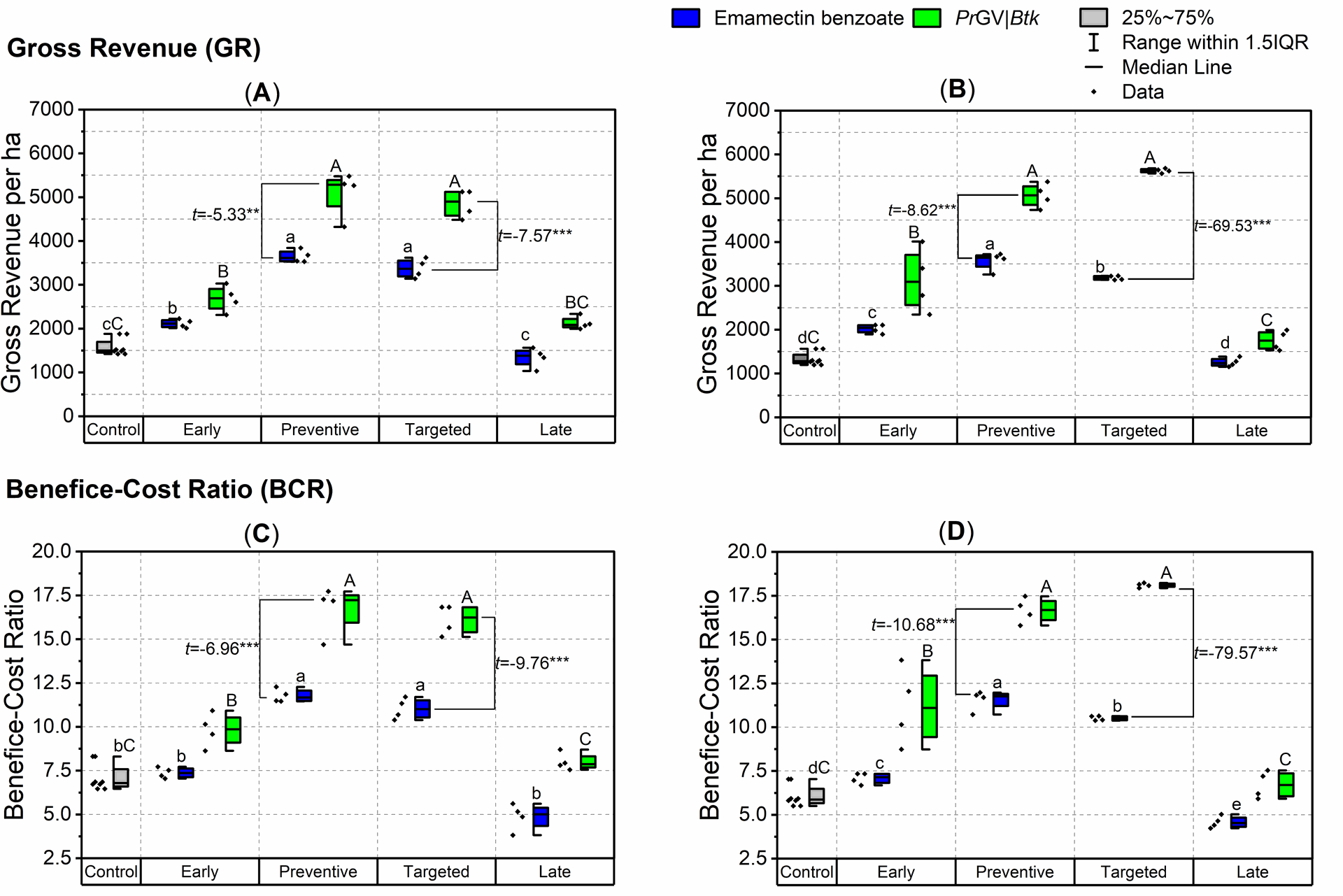
Economic analysis of insecticide application timing and frequency. The half boxes represent the interquartile range (25-75% of the data), while the whiskers delineate the outliers. Panels (A) and (B) display the gross revenues from the first and second experiments, respectively. Panels (C) and (D) showcase the benefit-cost ratio (BCR). The data on gross revenues and BCR underwent analysis via One-Way Analysis of Variance (ANOVA) with a significance level set at α = 5%. Additionally, a t-Test was utilized to compare two samples. The small letters positioned above the whiskers denote differences between emamectin benzoate-treated maize plants, whereas capital letters signify differences between PrGV|Btk treated maize plants.
Discussion
Our research assessed the effectiveness, ecological impact, and economic viability of an emamectin benzoate-based insecticide and a microbial bioinsecticide comprising Pieris rapae Granulovirus and Bacillus thuringiensis subsp. kurstaki (PrGV|Btk) in controlling fall armyworm (FAW) larvae. Our findings demonstrated that both insecticide formulations effectively mitigated FAW larval infestation across two distinct on-station experimental setups, aligning with previous studies (Deshmukh et al., 2020; Nboyine et al., 2022; Agboyi et al., 2023; Fiaboe et al., 2023a, 2024). While previous research has primarily focused on the bioefficacy of these insecticides against FAW, our study addressed the variation in timing and frequency of application, which has been less explored.
We observed that differences in treatment performance became notably apparent from the V7 growth stage of maize plants. Treatments receiving multiple applications, particularly at the early stage of crop growth, exhibited significant reductions in FAW larval numbers per plant. This emphasizes the importance of early insecticide application, as it targets smaller larvae and mitigates the development of larger, more damaging larvae later in the season (van den Berg et al., 2021; McClure et al., 2023).
Our analysis revealed that at least one insecticidal application at the early stage of crop growth resulted in less leaf damage by FAW between V7 to V9 growth stages. The "Early," "Preventive," and "Targeted" applications significantly reduced FAW-induced damage compared to the control, with the "Preventive" and "Targeted" treatments showing the most substantial impact. This difference can be attributed to the frequency of applications, with "Preventive" plots benefiting from three applications by the V9 stage and "Targeted" plots receiving two to three applications during both experiments. This underscores the critical role of both timing and frequency of applications in managing FAW damage effectively (Tang et al., 2010; van den Berg et al., 2021; Edde, 2022; McClure et al., 2023).
Initiating insecticide treatments during the early growth phases of maize and coupling them with subsequent applications can effectively limit damage within economically acceptable levels. "Targeted" approaches, tailored to minimize damage during critical growth stages, offer a promising alternative to standard preventive measures by reducing the need for frequent applications while still effectively controlling FAW infestations. This aligns with findings by van den Berg et al. (2021), who highlighted the importance of minimizing FAW damage from V5 to V9 stages in enhancing yield. While our study showed the efficacy of at least two insecticidal applications from V3 to V9 maize stage, the ecological implication of the doing is important.
Regarding parasitism results, our study observed that ceasing insecticide treatments at an early crop stage led to higher parasitization rates of FAW larvae, particularly evident in the first experiment. In the second trial, the beneficial impact of halting early treatments was overshadowed by the initial use of emamectin benzoate. In contrast, the early application of PrGV|Btk maintained moderate parasitism rates. This suggests that the strategic cessation or selection of insecticides can influence the biological control of FAW larvae, with implications for integrated pest management strategies that balance chemical control with biological conservation (Ratto et al., 2022). This aligns with recommendations by Agboyi et al. (2023) who recommended the use of bioinsecticides, including PrGV|Btk over chemical formulations, including emamectin benzoate for FAW control. Nevertheless, they also caution about the variable effectiveness of PrGV|Btk, noting lower parasitism rates in certain regions within their study scope. Conversely, Fiaboe et al. (2024) found no adverse effects on populations of natural enemies when integrating the use of this bioinsecticide with soil amendments applied in divided, smaller quantities, highlighting the potential for synergistic strategies in pest management.
Interestingly, our research also points to elevated parasitism rates at the V7 to V9 stages, particularly when damage-targeted insecticide applications, predominantly PrGV|Btk, were employed. Also, our PLS-DA analysis indicated that "Targeted" insecticide application correlated with increased parasitism at the V7 stage, underscoring the ecological benefits of this application strategy. Given the observed decrease in infestation levels with “targeted” insecticide application regimen, it is plausible to consider this approach both ecologically beneficial and conducive to enhanced maize yields. This is corroborated by Chisonga et al. (2023), who assert that minimizing FAW infestations during the V7 to V9 growth stages is crucial for optimizing maize yield outcomes.
In our investigation, we observed that both preventive and targeted applications of insecticide led to the highest maize grain yield, regardless of the specific insecticide used. This outcome underscores the significance of implementing at least two insecticidal applications between the V3-V9 stage of maize crop growth. To mitigate additional costs, we propose adopting infestation-tracked application strategies. As guided by (Prasanna et al., 2018), scouting for FAW management in maize involves systematic field inspections to monitor larval activity of the pest and the presence of its egg masses on plants. This method of scouting is recognized as an economical IPM strategy, allowing for the application of insecticides solely when it is deemed necessary, as supported by van den Berg et al. (2021). However, in our study, we found that the targeted application did not differ economically form preventive application.
While targeted applications are conceptually more efficient and aligned with IPM principles, their economic advantage over preventive applications may not always be realized due to the complexities of pest management, the inherent costs of application, and the challenges associated with accurately predicting and responding to pest pressures. This underscores the need for a nuanced understanding of pest ecology, application costs, and the effectiveness of scouting and threshold-based decision-making in the economic evaluation of insecticide application strategies in maize cultivation. Moreover, it is important to consider that factors beyond the direct costs of insecticide applications could influence the economic comparison of these strategies.
In our study, we found that the efficiency of targeted application though higher for the preventive and targeted treatments, differ significantly between insecticides. Targeted PrGV|Btk application resulted in highest benefice-cost than emamectin benzoate. The findings from our study reveal a nuanced landscape in the efficacy and economic viability of different insecticide treatments for maize, particularly when comparing targeted applications of PrGV|Btk (a biological control agent) and emamectin benzoate (a synthetic insecticide). The superior cost-benefit ratio observed with targeted applications of PrGV|Btk over emamectin benzoate can be attributed to a combination of factors related to yield impacts and market pricing for crops treated with synthetic versus biological insecticides. The differential efficiency between these insecticides under targeted application regimes underscores the importance of selecting the appropriate pest management strategy based on the specific pest pressure, crop stage, and the mode of action of the insecticide. Biological control agents like PrGV|Btk are often favored in IPM strategies due to their ecological outcomes, reduced environmental impact, and the lower risk of developing pest resistance compared to synthetic chemicals (Torres & Bueno, 2018; Akutse et al., 2020).
The higher benefit-cost ratio for PrGV|Btk may also reflect market preferences for maize produced with biological control methods, which can command a premium price due to increasing consumer demand for sustainably produced food. This price differential can significantly impact the economic analysis, making biological treatments more appealing from a financial perspective, despite potentially higher upfront costs or lower efficacy in some cases (Somasundram et al., 2016; Meemken & Qaim, 2018).
Moreover, the yield impact of using biological versus synthetic insecticides is a critical factor. If PrGV|Btk effectively controls the pest with minimal negative impact on the maize crop, the yield preserved coupled with a premium market price for biologically treated produce can lead to a higher overall economic return. In contrast, while synthetic insecticides like emamectin benzoate may offer potent for controlling FAW, as observed in this study, potential negative perceptions and market penalties for synthetic chemical use could offset these benefits (Pumarega et al., 2017).
These findings highlight the complexity of choosing between biological and synthetic pest control methods. The decision should consider not only the immediate efficacy of the pest control but also the broader implications for crop yield, market pricing, and consumer preferences. This holistic approach ensures that the selected pest management strategy aligns with both agronomic and economic goals, particularly in the context of sustainable agriculture practices.
Conclusion
These findings highlight the complexity of choosing be-tween biological and synthetic pest control methods. The decision should consider not only the immediate efficacy of the pest control but also the broader implications for crop yield, market pricing, and consumer preferences. The holistic approach ensures that the selected pest management strategy aligns with both agronomic, ecological and economic goals, particularly in the context of sustainable agriculture practices.
Declarations
Ethical statement
The study was conducted outside national parks and protected areas. In the Republic of Togo, neither the insect pest Spodoptera frugiperda nor its natural enemies are classified as endangered or protected under specific conservation regulations.
Funding
The authors received no specific funding for this work.
Conflict of interest
The authors affirm that there are no competing interests associated with this work.
Data availability
The authors will provide the raw data that supports the conclusions of this article, readily and without unnecessary reservation.
Acknowledgements
We express our sincere thanks to the technicians at the Entomology and Nematology Lab, School of Agronomy at the University of Lomé for their assistance in various agronomic activities during the study. Our gratitude also extends to all internal and external reviewers of our manuscript.
Author Information
Kokou Rodrigue Fiaboe
- Ecole Supérieure d’Agronomie (ESA), Université de Lomé, P.O. Box 1515-01, Lomé, Togo.
- International Centre of Insect Physiology and Ecology (icipe), P.O. Box 30772-00100, Nairobi, Kenya.
- Department of Zoology and Entomology, University of Pretoria, Private Bag X20, Hatfield 0028, South Africa.
Contribution: Conceptualization, Project administration, Investigation, Formal analysis, Visualization, Methodology, Writing original draft, Writing - Review and Editing.
For correspondence: rfiaboe@yahoo.com
Competing interests: No competing interests declared.
 0000-0001-9239-194X
0000-0001-9239-194X
Faicedois Abalo
- Ecole Supérieure d’Agronomie (ESA), Université de Lomé, P.O. Box 1515-01, Lomé, Togo.
Contribution: Investigation, Methodology, Writing original draft.
Competing interests: No competing interests declared.
 0009-0004-7176-8903
0009-0004-7176-8903
Kodjo Médard Abalo
- Ecole Supérieure d’Agronomie (ESA), Université de Lomé, P.O. Box 1515-01, Lomé, Togo.
Contribution: Investigation, Methodology, Writing original draft.
Competing interests: No competing interests declared.
 0009-0006-7760-1631
0009-0006-7760-1631
Emmanuel Peter
- International Centre of Insect Physiology and Ecology (icipe), P.O. Box 30772-00100, Nairobi, Kenya.
- Department of Zoology and Entomology, University of Pretoria, Private Bag X20, Hatfield 0028, South Africa.
- Department of Agronomy, Federal University Gashua, P.M.B 1005, Yobe State, Nigeria.
Contribution: Writing - Review and Editing.
Competing interests: No competing interests declared.
 0000-0003-2660-3830
0000-0003-2660-3830
Agnamto Ossara Agnamba
- Ecole Supérieure d’Agronomie (ESA), Université de Lomé, P.O. Box 1515-01, Lomé, Togo.
Contribution: Conceptualization, Project administration, Writing - Review and Editing.
Competing interests: No competing interests declared.
 0000-0002-3226-4823
0000-0002-3226-4823
Afoulélou Aboulaye
- Department of Agricultural Economics, University of Nairobi, P.O. Box 29053-00625, Nairobi, Kenya.
Contribution: Data Curation, Formal Analysis, Writing - Review and Editing.
Competing interests: No competing interests declared.
 0000-0001-9570-8826
0000-0001-9570-8826
Djima Koffi
- Ecole Supérieure d’Agronomie (ESA), Université de Lomé, P.O. Box 1515-01, Lomé, Togo.
- Division of Agricultural Entomology, Georg-August-Universität Göttingen, Germany.
Contribution: Conceptualization, Project administration, Writing - Review and Editing.
Competing interests: No competing interests declared.
 0000-0002-5698-2862
0000-0002-5698-2862
Komi Agboka
- Ecole Supérieure d’Agronomie (ESA), Université de Lomé, P.O. Box 1515-01, Lomé, Togo.
- West African Science Service Centre on Climate Change and Adapted Land Use (WASCAL), Université de Lomé, P.O. Box 1515-01, Lomé, Togo.
Contribution: Conceptualization, Project administration, Supervision, Writing - Review and Editing.
Competing interests: No competing interests declared.
 0000-0002-5920-3908
0000-0002-5920-3908
Reviewing Editor
Mawufe Komi Agbodzavu
- International Institute of Tropical Agriculture (IITA), Kinshasa, CD.
 0000-0001-5435-1250
0000-0001-5435-1250
Figures
Figure 1 | Effect of insecticide treatments on Spodoptera frugiperda-related damage in maize at various growth stages during the first on-station experiment. The bars display the mean±SE damage coefficients, which quantify the extent of damage inflicted by FAW larvae on maize plants at different developmental stages. The damage coefficients are normalized by dividing each plant's damage score (Davis et al., 1992) by the highest score recorded at a particular growth stage. The abbreviations Em., PrGV, and Btk denote emamectin benzoate, Pieris rapae Granulovirus, and Bacillus thuringiensis subsp. kurstaki, respectively. The analysis was conducted using One-Way Analysis of Variance (ANOVA) with a significance level set at α = 5%. The statistical significance of the results is denoted by asterisks, with * indicating P < .05, ** indicating P < .01, and *** indicating P < .001.
Figure 2 | Effect of Insecticide treatments on Spodoptera frugiperda-related damage in maize at various growth stages during the first on-station experiment. The bars display the mean±SE damage coefficients, which quantify the extent of damage inflicted by FAW larvae on maize plants at different developmental stages. The damage coefficients are normalized by dividing each plant's damage score (Davis et al., 1992) by the highest score recorded at a particular growth stage. The abbreviations Em., PrGV, and Btk denote emamectin benzoate, Pieris rapae Granulovirus, and Bacillus thuringiensis subsp. kurstaki, respectively. The analysis was conducted using One-Way Analysis of Variance (ANOVA) with a significance level set at α = 5%. The significance of the results is denoted by asterisks, with * indicating P < .05, ** indicating P < .01, and *** indicating P < .001, highlighting the statistical relevance of the observed differences.
Figure 3 | Effect of insecticide treatments on parasitism rates of FAW larvae. This figure illustrates the changes in parasitism rates of FAW larvae among various treatment plots throughout the maize plant growth stages. The lines trace the parasitism dynamics, reflecting how different insecticide applications influence the interaction between FAW larvae and their parasitoids over time. For specifics on when insecticides were applied for each treatment group, see Figure S1. The abbreviations PrGV and Btk stand for Pieris rapae Granulovirus and Bacillus thuringiensis subsp. kurstaki, respectively, indicating the types of bioinsecticides evaluated in this study.
Figure 4 | PLS-DA Biplot of Insecticide Application Timings on FAW Management. This biplot illustrates the associations between different insecticide application strategies ('Late', 'Control', 'Preventive', 'Targeted', and 'Early') and key biological and ecological variables in the context of Fall Armyworm (FAW) control. The axes represent the first (wc1) and second (wc2) latent variables of the PLS-DA model, explaining 34% of the total variance.
Figure 5 | Effects of insecticide on the maize grain yield. Bars depict the mean±SE maize grain yield. The bars represent the average maize grain yield (±SE) across different treatment plots, with yields extrapolated from measurements of 10 plants per plot to tons per hectare (t/ha). The abbreviations PrGV and Btk represent Pieris rapae Granulovirus and Bacillus thuringiensis subsp. kurstaki, respectively, indicating the specific bioinsecticides evaluated. Yield data were analyzed using One-Way Analysis of Variance (ANOVA), with a 5% significance threshold (α = .05). The effect of each insecticide treatment on yield, relative to the untreated control, is highlighted by asterisks: * for P < .05, ** for P < .01, and *** for P < .001, signifying varying levels of statistical significance. The notation 'ns' indicates no significant difference from the control.
Figure 6 | Economic analysis of insecticide application timing and frequency. The half boxes represent the interquartile range (25-75% of the data), while the whiskers delineate the outliers. Panels (A) and (B) display the gross revenues from the first and second experiments, respectively. Panels (C) and (D) showcase the benefit-cost ratio (BCR). The data on gross revenues and BCR underwent analysis via One-Way Analysis of Variance (ANOVA) with a significance level set at α = 5%. Additionally, a t-Test was utilized to compare two samples. The small letters positioned above the whiskers denote differences between emamectin benzoate-treated maize plants, whereas capital letters signify differences between PrGV|Btk-treated maize plants.
Abang AF, Nanga SN, Kuate AF, Kouebou C, Suh C, Masso C, Saethre MG, Mokpokpo Fiaboe KK. 2021. Natural enemies of fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) in different agro-ecologies. Insects 12: 509.
https://doi.org/10.3390/insects12060509 • Google Scholar
Adem M, Azadi H, Spalevic V, Pietrzykowski M,
Scheffran J. 2023.
Impact of integrated soil fertility management practices on maize yield in
Ethiopia. Soil and Tillage Research 227: 105595.
https://doi.org/10.1016/j.still.2022.105595
• Google Scholar
Agbodzavu MK, Lagat ZO, Gikungu M, Rwomushana I, Ekesi S, Fiaboe KKM. 2018. Performance of the newly identified endoparasitoid Cotesia icipe Fernandez-Triana & Fiaboe on Spodoptera littoralis (Boisduval). Journal of Applied Entomology 142: 646–653.
https://doi.org/10.1111/jen.12514
•
Google Scholar
Agboyi LK, Nboyine JA, Asamani E, Beseh P, Badii BK,
Kenis M, Babendreier D. 2023. Comparative effects of biopesticides on fall armyworm
management and larval parasitism rates in Northern Ghana. Journal of Pest
Science 96: 1417–1428.
https://doi.org/10.1007/s10340-023-01590-z
• Google Scholar
Akutse KS, Kimemia JW, Ekesi S, Khamis FM, Ombura OL,
Subramanian S. 2019.
Ovicidal effects of entomopathogenic fungal isolates on the invasive Fall
armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). Journal of
Applied Entomology 143: 626–634.
https://doi.org/10.1111/jen.12634
•
Google Scholar
Akutse KS, Subramanian S, Maniania NK, Dubois T, Ekesi
S. 2020.
Biopesticide research and product development in Africa for sustainable
agriculture and food security – experiences from the International Centre of
Insect Physiology and Ecology (icipe). Frontiers in Sustainable Food Systems
4: 563016.
https://doi.org/10.3389/fsufs.2020.563016
•
Google Scholar
Alene AD, Menkir A, Ajala SO, Badu-Apraku B,
Olanrewaju AS, Manyong VM, Ndiaye A. 2009. The economic and poverty impacts of maize
research in West and Central Africa. Agricultural Economics 40:
535–550.
https://doi.org/10.1111/j.1574-0862.2009.00396.x
•
Google Scholar
Aniwanou CTS, Sinzogan AAC, Deguenon JM, Sikirou R,
Stewart DA, Ahanchede A. 2021. Bio-efficacy of diatomaceous earth, household soaps, and
neem oil against Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae in
Benin. Insect 12: 18.
https://doi.org/10.3390/insects12010018
•
PubMed
• Google Scholar
Babendreier D, Koku Agboyi L, Beseh P, Osae M, Nboyine
J, Ofori SEK, Frimpong JO, Attuquaye Clottey V, Kenis M. 2020. The efficacy of
alternative, environmentally friendly plant protection measures for control of
Fall armyworm, Spodoptera frugiperda, in maize. Insects 11:
240.
https://doi.org/10.3390/insects11040240
•
Pubmed
•
Google Scholar
van den Berg J, Britz C, du Plessis H. 2021. Maize yield response
to chemical control of Spodoptera frugiperda at different plant growth
stages in South Africa. Agriculture (Switzerland) 11: 826.
https://doi.org/10.3390/agriculture11090826
•
Google Scholar
Chawanda G, Tembo YLB, Donga TK, Kabambe VH, Stevenson
PC, Belmain SR. 2023.
Agroecological management of fall armyworm using soil and botanical treatments
reduces crop damage and increases maize yield. Frontiers in Agronomy 5: 1114496.
https://doi.org/10.3389/fagro.2023.1114496
•
Google Scholar
Chisonga C, Chipabika G, Sohati PH, Harrison RD. 2023. Understanding the
impact of fall armyworm (Spodoptera frugiperda J. E. Smith) leaf damage
on maize yields. PLoS ONE 18: e0279138.
https://doi.org/10.1371/journal.pone.0279138
•
PubMed
• Google Scholar
Chivenge P, Mabhaudhi T, Modi AT, Mafongoya P. 2015. The potential role of
neglected and underutilised crop species as future crops under water scarce
conditions in Sub-Saharan Africa. International Journal of Environmental
Research and Public Health 12: 5685–5711.
https://doi.org/10.3390/ijerph120605685
•
PubMed
•
Google Scholar
Davis F, Ng S, Williams W. 1992. Visual rating scales
for screening whorl-stage corn for resistance to fall armyworm. Technical
Bulletin-Mississippi Agricultural and Forestry Experiment Station 186:
1–9.
Google Scholar
Deshmukh S, Pavithra HB, Kalleshwaraswamy CM, Shivanna
BK, Maruthi MS, Mota-Sanchez D. 2020. Field efficacy of insecticides for management
of invasive fall armyworm, Spodoptera frugiperda (J. E. Smith)
(Lepidoptera: Noctuidae) on maize in India. Florida Entomologist 103:
221–227.
https://doi.org/10.1653/024.103.0211
•
Google Scholar
Edde PA. 2022. Arthropod pests of pulses. In: Edde PA, ed.
Field Crop Arthropod Pests of Economic Importance. Academic Press Inc.,
612–682.
https://doi.org/10.1016/B978-0-12-818621-3.00002-1
•
Google Scholar
Erenstein O, Jaleta M, Sonder K, Mottaleb K, Prasanna
BM. 2022.
Global maize production, consumption and trade: trends and R&D
implications. Food Security 14: 1295–1319.
https://doi.org/10.1007/s12571-022-01288-7
•
Google Scholar
Fiaboe KR, Agboka K, Agnamba AO, Teyo KL, Amegah AL,
Koffi D, Kpadonou GE, Agboka KM, Gwokyalya R, Fening KO, et al. 2024.
Fertilizer-bioinsecticide synergy improves maize resilience to Spodoptera
frugiperda infestation. Crop Protection 177: 106548.
https://doi.org/10.1016/j.cropro.2023.106548
•
Google Scholar
Fiaboe KR, Fening KO, Gbewonyo WSK, Deshmukh S. 2023a. Bionomic responses of
Spodoptera frugiperda (J. E. Smith) to lethal and sublethal concentrations of
selected insecticides. Plos One 18: e0290390.
https://doi.org/10.1371/journal.pone.0290390
•
PubMed
•
Google Scholar
Fiaboe KR, Yusuf AA,
Torto B, Khamis FM. 2023b. Herbivore intraguild
interactions decrease ectoparasitoid Bracon nigricans parasitism of Phthorimaea
absoluta and responses to tomato volatiles. Frontiers in Ecology and
Evolution 11: 1200040.
https://doi.org/10.3389/fevo.2023.1200040
•
Google Scholar
Goergen G, Kumar PL, Sankung SB, Togola A, Tamò M. 2016. First report of
outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera,
Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 11:
e0165632.
https://doi.org/10.1371/journal.pone.0165632 •
PubMed
•
Google Scholar
Habte E, Marenya
P, Beyene F, Bekele A. 2023. Reducing susceptibility to drought
under growing conditions as set by farmers: The impact of new generation
drought tolerant maize varieties in Uganda. Frontiers in Sustainable Food
Systems 6: 854856.
https://doi.org/10.3389/fsufs.2022.854856 •
Google Scholar
Infante PA, Moore KJ, Lenssen AW, Archontoulis S V.,
Scott P, Fei S zhang.
2018. Phenology and biomass production of adapted and non-adapted
tropical corn populations in central Iowa. Agronomy Journal 110:
171–182.
https://doi.org/10.2134/agronj2016.11.0666 •
Google Scholar
Koffi D, Agboka K, Adenka DK, Osae M, Tounou AK, Anani
Adjevi MK, Fening KO, Fening KO, Meagher RL. 2020. Maize infestation of fall armyworm
(Lepidoptera: Noctuidae) within agro-ecological zones of Togo and Ghana in West
Africa 3 yr after its invasion. Environmental Entomology 49:
645–650.
https://doi.org/10.1093/ee/nvaa048 •
PubMed •
Google Scholar
Koffi D, Kyerematen R, Eziah VY, Osei-Mensah YO,
Afreh-Nuamah K, Aboagye E, Osae M, Meagher RL. 2021. Assessment of impacts of fall armyworm,
Spodoptera frugiperda (Lepidoptera: Noctuidae) on maize production in Ghana. Journal
of Integrated Pest Management 11: 20.
https://doi.org/10.1093/jipm/pmaa015 •
Google Scholar
Koffi D, Agboka K,
Adenka DK, Osae M, Tounou AK, Anani Adjevi MK, Fening KO, Fening KO, Meagher RL. 2020a. Maize
infestation of Fall Armyworm (Lepidoptera: Noctuidae) within agro-ecological
zones of Togo and Ghana in West Africa 3 Yr after its invasion. Environmental
Entomology 49: 645–650.
https://doi.org/10.1093/ee/nvaa048 •
Google Scholar
Koffi D, Agboka K,
Adjevi MKA, Adom M, Tounou AK, Meagher RL. 2023a. The
natural control agents of the fall armyworm, Spodoptera frugiperda in
Togo: moderating insecticide applications for natural control of the pest? Journal
of Pest Science 96: 1405–1416.
https://doi.org/10.1007/s10340-023-01662-0
•
Google Scholar
Koffi D, Agboka K,
Fening KO, Adjevi MKA, Badziklou JEA, Tchegueni M, Tchao M, Meagher RL. 2023b. Spodoptera
frugiperda in Togo 5 years on: Early impact of the invasion and future
developments. Bulletin of Entomological Research 113: 21–28.
https://doi.org/10.1017/S0007485322000207 •
Google Scholar
Koffi D, Kyerematen R,
Eziah VY, Agboka K, Adom M, Goergen G, Meagher RL. 2020b.
Natural enemies of the Fall armyworm, Spodoptera frugiperda (J.E. Smith)
(Lepidoptera: Noctuidae) in Ghana. Florida Entomologist 103: 85.
https://doi.org/10.1653/024.103.0414 • Google Scholar
Koffi D, Kyerematen R,
Eziah VY, Osei-Mensah YO, Afreh-Nuamah K, Aboagye E, Osae M, Meagher RL. 2021.
Assessment of impacts of fall armyworm, Spodoptera frugiperda
(Lepidoptera: Noctuidae) on maize production in Ghana. Journal of Integrated
Pest Management 11: 20.
https://doi.org/10.1093/jipm/pmaa015 •
Google Scholar
Mauki C, Jeckoniah J,
Massawe GD. 2023. Smallholder rice farmers profitability in
Agricultural Marketing Co-operative Societies in Tanzania: A case of Mvomero
and Mbarali districts. Heliyon 9: e17039.
https://doi.org/10.1016/j.heliyon.2023.e17039 • PubMed
•
Google Scholar
McClure M, Herreid J, Jabbour R. 2023. Insecticide
application timing effects on alfalfa insect communities. Journal of
Economic Entomology 116: 815–822.
https://doi.org/10.1093/jee/toad071 •
PubMed
•
Google Scholar
Meemken EM, Qaim M. 2018. Organic Agriculture, Food Security, and
the Environment. Annual Review of Resource Economics 10: 39–63.
https://doi.org/10.1146/annurev-resource-100517-023252
• Google Scholar
Midega CAO, Pittchar JO, Pickett JA, Hailu GW, Khan ZR. 2018. A climate-adapted
push-pull system effectively controls fall armyworm, Spodoptera frugiperda (J E
Smith), in maize in East Africa. Crop Protection 105: 10–15.
https://doi.org/10.1016/j.cropro.2017.11.003 •
Google Sholar
Montezano DG, Specht A, Sosa-Gómez DR, Roque-Specht
VF, Sousa-Silva JC, Paula-Moraes S V., Peterson JA, Hunt TE. 2018. Host plants of Spodoptera
frugiperda (Lepidoptera: Noctuidae) in the Americas. African Entomology
26: 286–300.
https://doi.org/10.4001/003.026.0286 •
Google Scholar
Nboyine JA, Asamani E, Agboyi LK, Yahaya I, Kusi F,
Adazebra G, Badii BK.
2022. Assessment of the optimal frequency of insecticide sprays required
to manage fall armyworm (Spodoptera frugiperda J.E Smith) in maize (Zea
mays L.) in northern Ghana. CABI Agriculture and Bioscience 3:
1–11.
https://doi.org/10.1186/s43170-021-00070-7 •
Google Scholar
Niassy S, Agbodzavu MK, Kimathi E, Mutune B,
Abdel-Rahman EFM, Salifu D, Hailu G, Belayneh YT, Felege E, Tonnang HEZ, et
al. 2021.
Bioecology of fall armyworm Spodoptera frugiperda (J. E. Smith), its management
and potential patterns of seasonal spread in Africa. PLoS ONE 16:
e0249042.
https://doi.org/10.1371/journal.pone.0249042 • PubMed
• Google Scholar
Otim MH, Fiaboe KKM, Akello J, Mudde B, Obonyom AT,
Bruce AY, Opio WA, Chinwada P, Hailu G, Paparu P. 2021. Managing a
transboundary pest: the fall armyworm on maize in Africa. In: Shields VDC, ed.
Moths and Caterpillars. Rijeka: IntechOpen, 13.
http://doi.org/10.5772/intechopen.96637 •
Google Scholar
Prasanna BM, Huesing JE, Eddy R, Peschke VM (eds). 2018. Fall armyworm in
Africa: A guide for intergrated pest management. : 120.
Google Scholar
Pumarega J, Larrea C, Muñoz A, Pallarès N, Gasull M,
Rodríguez G, Jariod M, Porta M. 2017. Citizens’ perceptions of the presence and
health risks of synthetic chemicals in food: results of an online survey in
Spain. Gaceta Sanitaria 31: 371–381.
https://doi.org/10.1016/j.gaceta.2017.03.012
• PubMed
• Google Scholar
R Core Team. 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Ratto F, Bruce T, Chipabika G, Mwamakamba S,
Mkandawire R, Khan Z, Mkindi A, Pittchar J, Sallu SM, Whitfield S, et al. 2022. Biological control
interventions reduce pest abundance and crop damage while maintaining natural
enemies in sub-Saharan Africa: A meta-analysis. Proceedings of the Royal
Society B: Biological Sciences 289: 20221695.
https://doi.org/10.1098%2Frspb.2022.1695
•
PubMed
•
Google Scholar
Signoretti AGC, Peñaflor MFGV, Bento JMS. 2012. Fall Armyworm, Spodoptera
frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), female moths respond to
herbivore-induced corn volatiles. Neotropical Entomology 41:
22–26.
https://doi.org/10.1007/s13744-011-0003-y
•
PubMed
•
Google Scholar
Somasundram C, Razali Z, Santhirasegaram V. 2016. A review on organic
food production in Malaysia. Horticulturae 2: 12.
https://doi.org/10.3390/horticulturae2030012
•
Google Scholar
Spark AN. 1979. A review of the biology of the fall armyworm. The
Florida Entomologist 62: 82–87.
https://doi.org/10.2307/3494083
•
Google Scholar
Tang S, Tang G, Cheke RA. 2010. Optimum timing for
integrated pest management: Modelling rates of pesticide application and
natural enemy releases. Journal of Theoretical Biology 264:
623–638.
https://doi.org/10.1016/j.jtbi.2010.02.034 •
PubMed
•
Google Scholar
Torres JB, Bueno A de F. 2018. Conservation
biological control using selective insecticides – A valuable tool for IPM. Biological
Control 126: 53–64.
https://doi.org/10.1016/j.biocontrol.2018.07.012 •
Google Scholar
Zhang BQ, Cheng R-L, Wang X-F, Zhang C-XZ. 2012. The Genome of Pieris rapae Granulovirus. Journal of Virology 86: 9544–9544.
https://doi.org/10.1128/jvi.01431-12 • PubMed • Google Scholar
